“Ideas, like living organisms, have their natural history, growing from conception through a more or less tumultuous adolescence and reproductive maturity to an old age, when they act as a bar to further progress. During this time they become so modified that their origin is obscured” Sir Richard Doll (1)
Looking at the occurrence of a disease in time and place, and assessing what might have influenced changes, is known as the science of epidemiology. The theory, that diverticular disease (DD) was caused by low levels of fibre in the diet, has been prominent for about 40 years. This was based on the rarity of DD in Uganda compared with Western countries such as Great Britain or the USA. It was assumed that high levels of fibre in the Ugandan diet protected people from DD and that an increase in dietary fibre would prevent DD and its symptoms would be eliminated. This was a conclusion too far. It ignored the rarity of DD in people eating very little fibre (2,3) and that vegetarians can get DD (4,5). There is no evidence that a high fibre diet prevents DD. The theory is so entrenched that if DD appears in a country then it is assumed that its inhabitants have changed from their normal to a low fibre western diet. This is particularly incongruous when applied to right-sided DD in the caecum and ascending colon. Even the theory’s originators thought low fibre levels could not be relevant to this area (6)
Data from post-mortems, mortality statistics and surveys can provide information on the occurrence of DD, each aspect contributing to the overall picture. Song et al. (7) showed how colonoscopy findings, over time, could plot a rising prevalence of DD in Korea. Jun and Stollman in 2002 (8) collected results from research papers on the % of patients with DD in series of examinations by colonoscopy or barium enema Xray. They used these results to show that changes in the prevalence of DD varied greatly in time and between countries. Searching through later research reports mainly in the PubMed website gives this type of information for many more countries. (References to these sources are too numerous to include here). The results fall into 4 distinct patterns of when DD appeared and how numbers have changed over time until 2010.
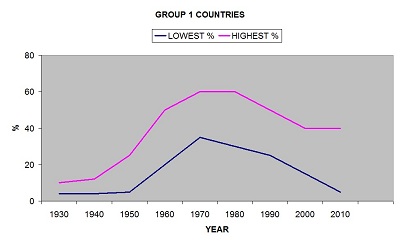
GROUP 1 AUSTRALIA, ENGLAND, USA, SWEDEN.DD appeared before the 1930’s, numbers rose to a peak about 1970-1980 and are falling since. Highest level about 60%
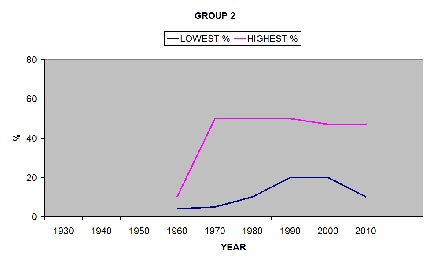
GROUP 2 FINLAND, NORWAY, GERMANY, ITALY, GREECE, NETHERLANDS. DD only appeared about 1960. There was variation between countries, some rose rapidly. Levels have been fairly stable or falling since about 1980. Highest level reached was 50%
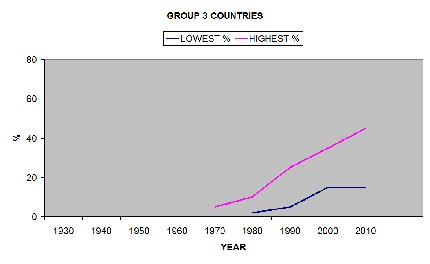
GROUP 3 INDIA, SINGAPORE, HONG KONG, JAPAN, BRAZIL, KOREA. DD first appeared about 1970 and numbers have been rising slowly since, reaching 45% in 2010.
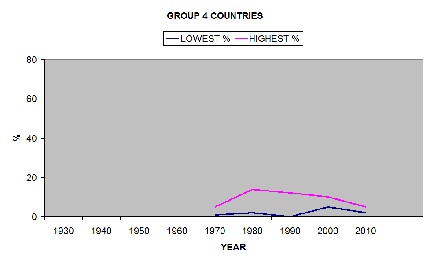
GROUP 4 KENYA, UGANDA, GHANA, NIGERIA, JORDAN, IRAN, PERU, CHINA. DD first appeared about 1970 but remained low or falling since, no results were over 15%.
There have always been differences in the prevalence of DD with claims that the level of dietary fibre was responsible. Emigration to a western country may (9,10) or may not (11,12) result in an increase in DD. Diverticulosis is still rare in China, but levels in younger Chinese in Hong Kong have not yet reached the levels in Caucasians (13,14). There are marked differences in DD levels within the country in Malaya (15), Singapore (16), Israel (17) and England (18). In these countries dietary fibre appears less relevant than racial, ethnic or perhaps even religious background.
Differences between urban and rural areas in developing countries have been observed for a long time. Higher counts of DD have been related to socio-economic status and city life, but the diet can be either vegetarian (19) or native (20). Alternatively, a change to a more Western diet has not resulted in an increase in DD prevalence (21).
The fibre theory was an inversion of Cleaves hypothesis (22) that refined carbohydrates in the diet were the cause of Western diseases. This inversion produced the assumption that dietary fibre protected against DD. Some reviewers now not happy with the fibre theory, have reversed to high levels of red meat or fat or the balance of colon bacteria as possible causes of DD. The fibre theory advocates should twist again. They should consider that the cause of DD lies in something introduced into Western lifestyle which was not present in a developing country. We know that DD is an acquired and not a hereditary disease, but it can be familial i.e. be present in a few generations of a family. A likely interaction between the body and its environment does not exclude a genetic influence on who would be affected.
In Western countries (Groups 1 & 2) the prevalence of DD appears to have peaked and is in slow decline. In Group 3 we get an insight into the beginning of the appearance of DD. Gender preponderance can vary without any dietary difference. There are reports of younger people being diagnosed, suggesting that people can grow old with DD rather than acquire it in old age. Growth of wealth and industry is a feature of this group. In contrast, countries in Group 4 appear to have restricted DD to few, urban wealthy people who perhaps have contact with the Western world. The cause of DD appears far more complicated than the fibre protection theory based on countries which were at the extremes of the worldwide DD epidemic 40 to 50 years ago. The epidemiology of DD has changed considerably since then.
THE % OF PATIENTS WITH DIVERTICULAR DISEASE IN COLON EXAMINATIONS
© Mary Griffiths 2011
NOTE This article was used in the August 2012 issue of the Journal of the Bladder and Bowel Foundation
REFERENCES
- Sir Richard Doll FRS. Foreword “Dietary fibre, fibre-depleted foods and disease” Trowell, Birkitt & Heaton Eds. Academic Press 1985.
- The Weston A Price Foundation. Out of Africa: what Drs. Price and Burkitt discovered in their studies of sub-Sahara tribes. https://www.westonprice.org
- Sinclair HM. Essential fatty acids and chronic degenerative diseases. In “Nutrition and killer diseases” Rose J Ed. 1982 Noyes New Jersey page 69.
- Crowe FL et al. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ 2011, 343, d4135.
- Gear JSS et al. Symptomless diverticular disease and intake of dietary fibre. Lancet 1979, 313, 511.
- Painter NS. Diverticular disease of the colon. Norgine Ltd. 1977, page 10. ISBN 0 901210 14 5.
- Song JH et al. Clinical characteristics of colon diverticulosis in Korea: a prospective study. Korean J Intern Med.2010, 25,140.
- Jun S & Stollman N. Epidemiology of diverticular disease. Best Practice and Research Clin Gastroenterol 2002, 16, 529.
- Sato E et al. Polyps and diverticulosis of large bowel in autopsy population of Akita prefecture with Miyagi. Cancer 1976, 37, 1316. Cited by Ref 8.
- Hjern F et al. Diverticular disease and migration – the influence of acculturation to a Western lifestyle on diverticular disease. Aliment Pharmacol Ther. 2006, 23, 797.
- Kang JY et al. Diverticular disease of the colon: ethnic differences in frequencies. Aliment Pharmacol Ther. 2004, 19, 765.
- Loffeld RJ. Diverticulosis of the colon is rare amongst immigrants living in the Zaanstreek region in the Netherlands. Colorectal Dis. 2005, 7, 559.
- Bai Y et al. Epidemiology of lower gastrointestinal bleeding in China: single-centre series and systemic analysis of Chinese literature with 53,951 patients. J Gastroenterol Hepatol. 2011, 26, 678.
- Chan CC et al. Colonic diverticulosis in Hong Kong: distribution pattern and clinical significance. Clin Radiol. 1998, 53, 842.
- Rajendra S et al. Colonic diverticular disease in a multiracial Asian patient population has an ethnic predilection. Eur J Gastroenterol Hepatol. 2005, 17, 871.
- Lee YS. Diverticular disease of the large bowel in Singapore. An autopsy survey. Dis Colon Rectum. 1986, 29,330.
- Levy N et al. The changing epidemiology of diverticular disease in Israel. Dis Colon Rectum, 1985, 28, 416.
- Golder M et al. Demographic determinants of risk, colon distribution and density scores of diverticular disease. World J Gastroenterol. 2011, 17, 1009.
- Goenka MK et al. Colonic diverticulosis in India: the changing scene. Indian J Gastroenterol. 1994, 13, 86.
- Archampong EQ. Diverticular disease in urban Kenyons. BMJ 1979, Sept 15, 672.
- Segal I et al. Persistent low prevalence of Western digestive diseases in Africa: confounding aetiological factors. Gut 2001, 48, 730.
- Cleave TL. The saccharine disease. Chap 3, Saccharine disease and the colon. John Wright & Sons, Bristol, 1974. (Available online at http://journeytoforever.org/farm_library/Cleave/ )
Tags: Diet, epidemiology, Fibre, Nicotine, Smoking, Statistics